Enterprise Risk Management
Quickly assess and take action on your organization’s biggest risks with a connected view of risks and controls.
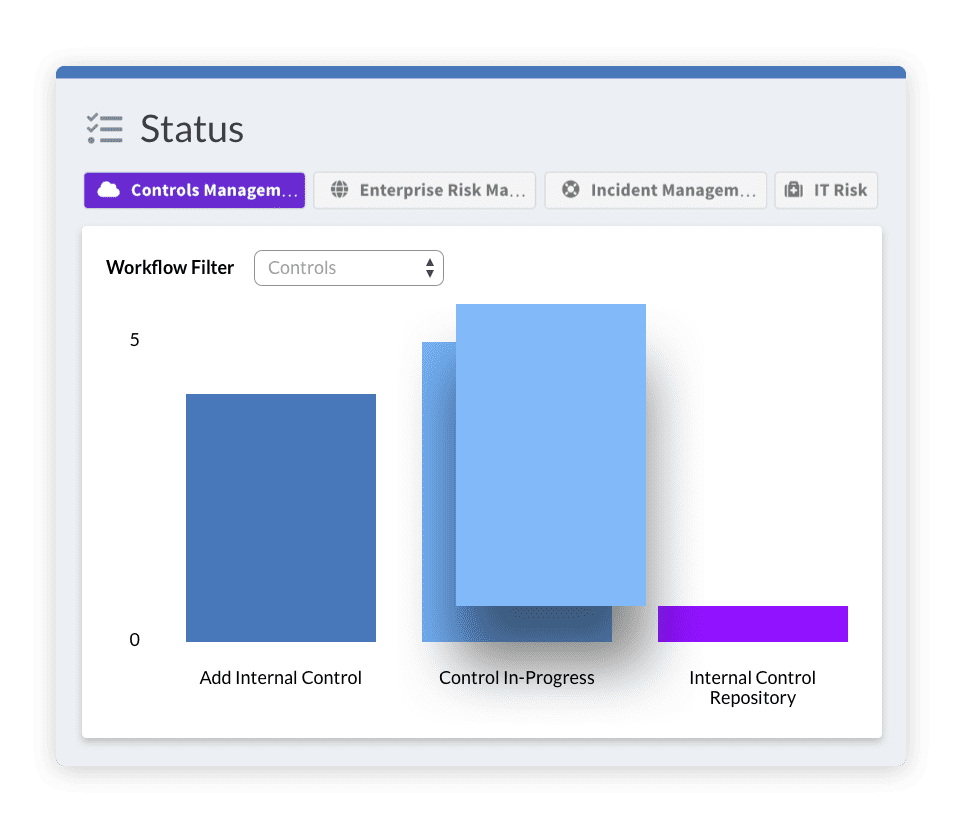
From the Food and Drug Administration (FDA) to the European Medicines Agency (EMA), agencies around the world are throwing down their own set of regulations for pharmaceutical, health sciences, and biotech firms. Using Risk Cloud’s drag-and-drop interface, you can quickly and easily build processes like field force monitoring and biosecurity assessments and remain compliant with all of them.
See For YourselfNot only are you up against those government regulations, you also need to adhere to industry standards like CGMP and ISO. With agile controls management, Risk Cloud ensures these best practices are met.
See the Difference with Risk Cloud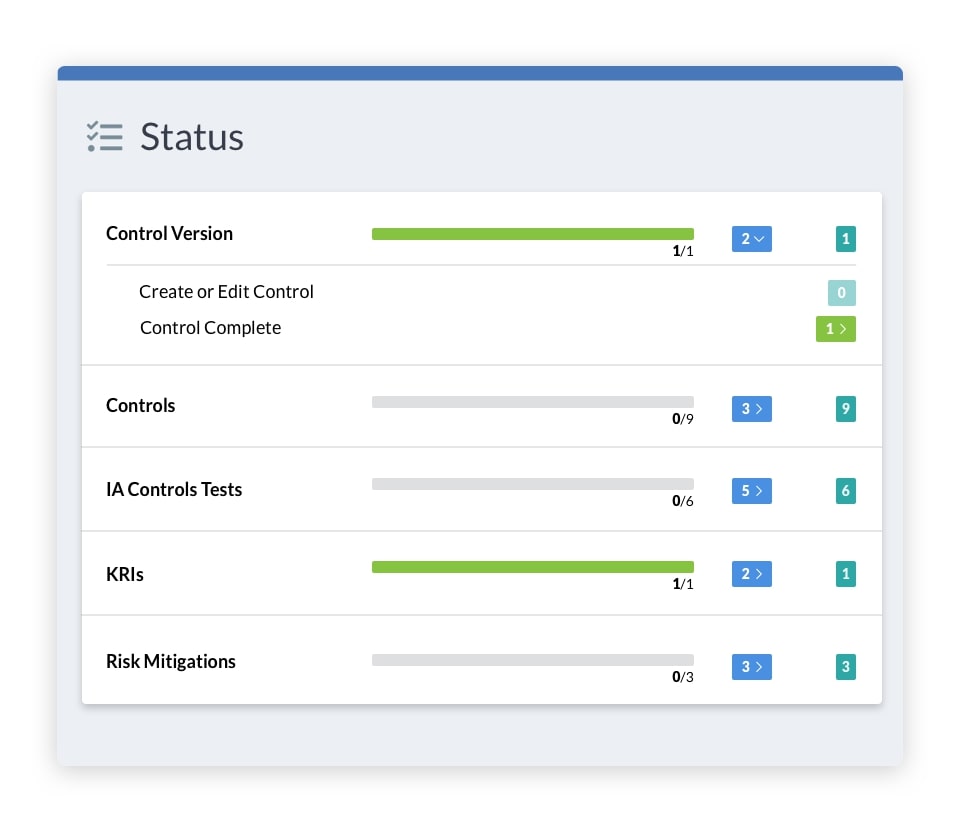
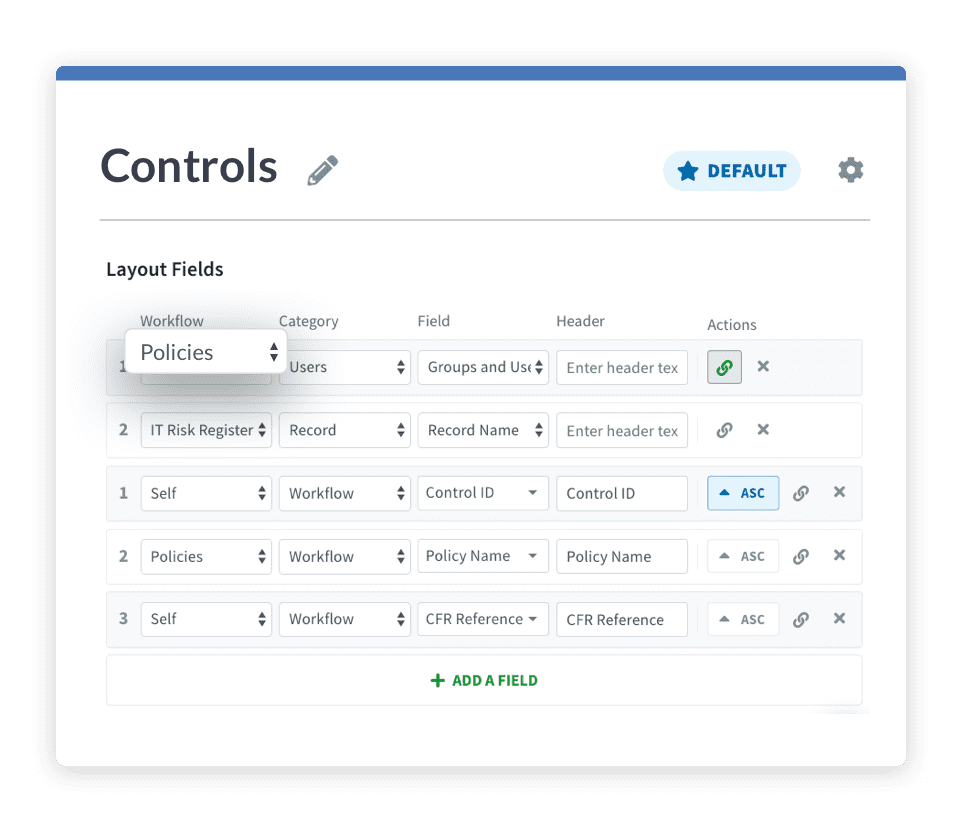
In the world of medicine, change is constant. But with Risk Cloud automation, you can identify, manage, and implement necessary updates to ensure you’re compliant and prepared for whatever the future holds.
View Compliance Management Brochure- Head of Risk and Compliance, Computer Software

Quickly assess and take action on your organization’s biggest risks with a connected view of risks and controls.
Identify, quantify, and prioritize cyber risks by linking vulnerabilities and threats to expected business impact.
Eliminate redundant controls and improve program efficiency by dynamically linking risks, controls, assessments, and evidence.
If you want your GRC engine to run smoothly, you need to look at systems holistically. Create a…
Share connected risk insights within the business context.
With the increasing reliance on digital infrastructure, regulatory bodies are stepping up to ensure these institutions are not…
The Network and Information Security Directive (NIS2) is ushering in new standards for cybersecurity across the European Union.…
With Risk Cloud, AI is not just a tool for speed but a catalyst for smarter and more…